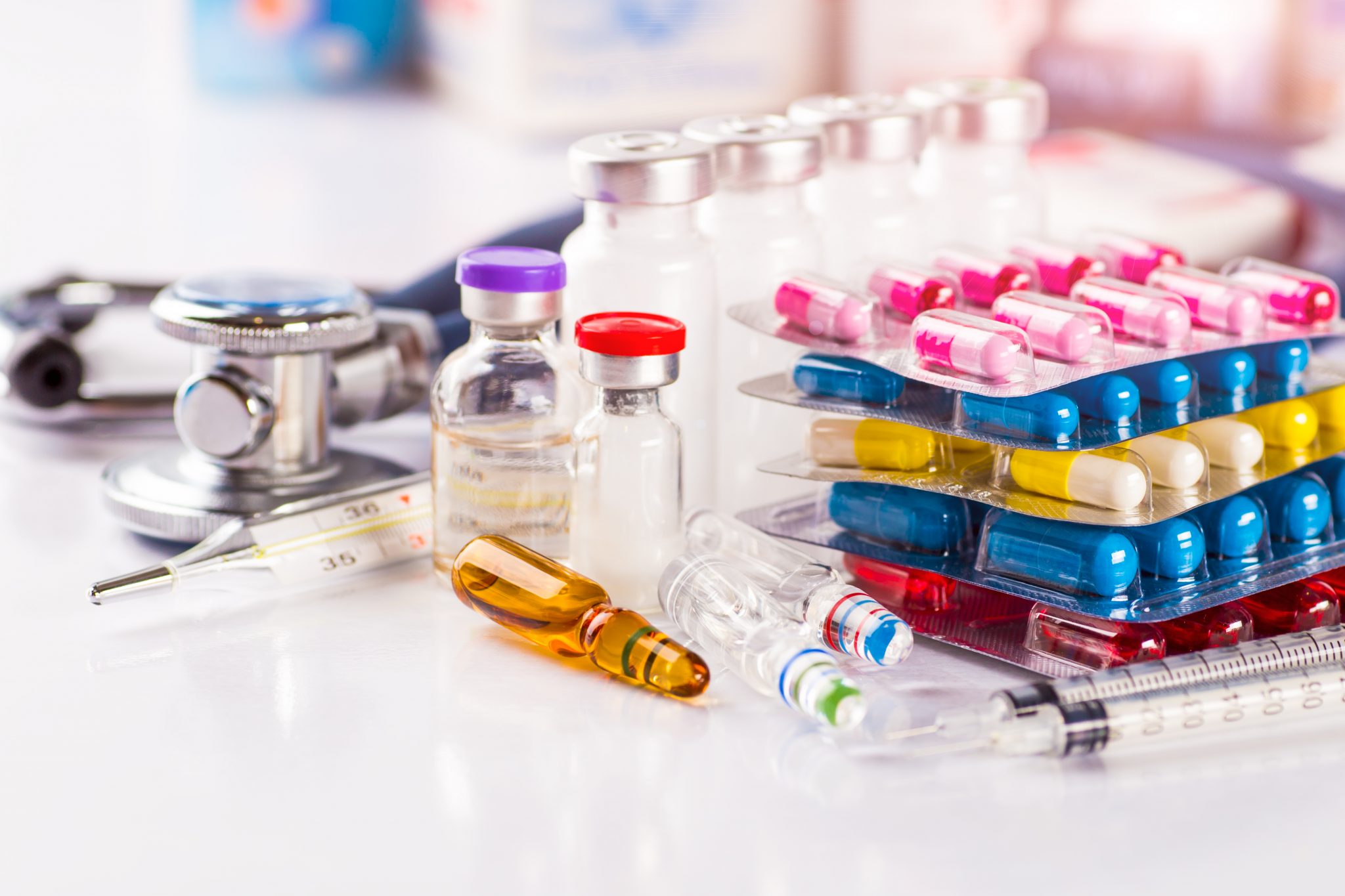
Greek patients are experiencing significant challenges in accessing the innovative pharmaceutical treatments they need, with just one out of five new pharmaceutical drugs available in the European Union and approved by the European Medicines Agency (EMA) reaching Greece, says In.gr.
Moreoever, even though some treatments have reached Greece, they are not accessible to everyone, according to a series of studies by the Hellenic Association of Pharmaceutical Companies (SFEE) and the European Federation of Pharmaceutical Industries and Associations (EFPIA), in collaboration with research firm IQVIA.
The studies investigate the waiting time for innovative treatments to reach Greece, the causes of delays, the availability of new medicines over the past decade, and the comparison of state participation in pharmaceutical expenditure among European countries and found that Greece spends 70% less on pharmaceuticals than the European average.
SFEE President Olympios Papadimitriou and General Manager Michalis Cheimonas emphasized that while funding needs to be increased, the Greek state needs to ensure equal, universal, and timely access to the new treatments that make it to Greece.
And funding and access must be matched with controls and digital tools to improve the investment performance of pharmaceuticals because, according to SFEE, the business environment for pharmaceutical companies in Greece is ‘unsustainable’ and less attractive for investment.
According to EFPIA’s study, Greece approves innovative pharmaceutical drugs faster than the European average. The average time for an innovative drug to become available in Greece is 587 days, which is 56 days faster than the European average.
However, despite availability from the first day of EMA approval, patient access remains limited.
Only 52% of reimbursed innovative drugs are fully available in Greece, with oncology drugs prioritized and combinations facing significant delays.
The main reasons for delays in Greece are the prolonged pricing and reimbursement processes and insufficient budget allocations for their distribution to the population.
Only one in five drugs approved in the last four years is reimbursed in Greece, with another 29% distributed and reimbursed through the Institute of Pharmaceutical Research and Technology (IFET) without their reimbursement being officially approved as not related request has ever been submitted.
Overall, the study showed that delays in the submission of applications in Western Europe are largely due to the requirements of the Health Technology Assessment process, whereas in Southern Europe, they are primarily attributed to a lack of financial viability, which affects commercial decisions regarding the release of these drugs in local markets.
Specifically for Greece, limited availability affects one in two new drugs, with the primary causes of delay being the lack of financial viability due to exorbitant mandatory rebates and clawbacks, the need for drugs to be evaluated and marketed in five out of eleven EU countries with assessment bodies, and the accessibility of available drugs depending on their distribution channel and the specifics of their approval process says In.gr.
Greece’s Funding Gap
Over the past decade, state funding for pharmaceutical care in Greece decreased by 14.9% while the average funding increase in Europe was 55.2%.
The funding shortfall severely impacts patient care, with per capita spending on medications in Greece significantly lower than in other European countries.
Specifically, the per capita spending on medications reaches 53 euros for hospital medications and 202 euros for outpatient medications in Greece.
Meanwhile in Southern Europe, the average per capita spending is 146 euros and 232 euros respectively, and in Western Europe, it rises to 178 euros and 370 euros, respectively.
Thus, patient coverage falls short by 64-70% compared to Southern and Western Europe for hospital pharmaceutical care and by 13-45% for outpatient pharmaceutical care, says In.gr.
Overall, the average per capita pharmaceutical expenditure both inside and outside hospitals reaches 255 euros, which is 32% below the Southern European average of 378 euros and 53% below the Western European average of 548 euros.
As a potential solution to the challenges faced by pharmaceutical companies in Greece, SFEE has proposed a multi-year cooperation memorandum between the state and the pharmaceutical industry to enhance predictability and transparency.
Source: tovima.com
Latest News

World Day of Physical Activity: About One in Three Greek Adolescents Physically Inactive
Almost one-third (30.4%) of Greek adolescents are considered physically inactive, meaning they engage in less than three days of physical activity per week. This rate is significantly higher than the international average of 24%

Unseasonably Low Temps, Storms, Snow Forecasted for Greece
A sharp decline in temperatures will begin Sunday afternoon in Macedonia and Thrace, progressively affecting central and southern Greece through Monday and Tuesday

Higher Prices for Easter Table Staples ‘Sting’ Greek Households
As inflation and production costs continue to weigh on the market, Greek families may find this year's Easter table more expensive than in previous year

Eluned Morgan: The Parthenon Marbles should be returned to Greece
The Welsh First Minister takes a clear stance in support of the reunification of the Parthenon Marbles in an exclusive interview with TA NEA Weekend

Traffic Disruptions in Athens This Weekend for Major Cycling Event
Athens police advise all residents and visitors to plan ahead and allow extra travel time

Trump’s 10% Tariffs Take Effect, Ushering in New Era in Trade
Trump’s tariffs represent a direct break from the post-WWII global trade framework that prioritized negotiated, reciprocal tariff reductions.

Oil Price Drops 8%
The sell-off was fueled by escalating fears of a global trade war after China responded aggressively to new U.S. tariffs, signaling a deepening economic rift.

Greece Opens Summer 2025 Season with 28.2 Million Airline Seats, Up 4.6%
The United Kingdom remains Greece’s largest source market, accounting for 5.6 million airline seats—an increase of 2.2% from last year—and representing 20% of the total.

Greece’s Top Unions Announce April 9 Nationwide Strike
As of now, there is no official announcement on whether workers in public transport systems will join the strike, leaving questions about the availability of buses, metro, and trams on the day.

PM Mitsotakis to Chair New Democracy’s Committee Meeting
Today’s meeting is seen as a crucial opportunity to halt internal disputes within ND and reaffirm unity within the party.




























![Δασμοί Τραμπ: Πώς οι επενδυτές μπορούν να ανταποκριθούν στην αναταραχή; [γραφήματα]](https://www.ot.gr/wp-content/uploads/2025/04/2025-04-02T215031Z_1105693786_RC2RPDAWF37N_RTRMADP_5_USA-TRUMP-TARIFFS-600x400.jpg)










 Αριθμός Πιστοποίησης
Αριθμός Πιστοποίησης